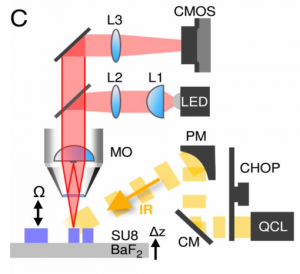
Adimec’s gentificTM High Full Well camera (Q-2HFW) was used to provide the digital images of the tissue, and in the above image, you can see where the camera (labelled CMOS) was used in the set-up of the IR-OH microscope.
Adimec spoke with Martin Schnell about his paper, “All-digital histopathology by infrared-optical hybrid microscopy” and the process he went through using the Q-2HFW.
- Where do you see the use of IR-OH tools to open more possibilities with microscopy?
Infrared microscopy (IR microscopy) will eventually be coming to a clinical setting and we are hoping to get the microscope to be an IR-OH microscope. The reason for this is because we want to reduce the workload for pathologists. Traditional IR detectors are not easy to run because the components of these detectors can absorb IR light. Having an optical microscope with the IR capabilities in the clinic makes looking at the morphology and biochemistry levels of the tissues much easier for the user.
- What advantages do you see using IR-OH compared to staining? The digital histopathology advantages.
When there is cancer in tissue, there is a change in the tissue morphology and these changes are what we want to easily identify. For more than a century, dyes have been used for viewing tissue morphology. However, we think that staining is an old technique and needs to be replaced. Staining takes a lot of training and time and it can be easy to use too little or too much dye when staining the tissue. That can make seeing changes in tissue morphology more difficult. With IR imaging, we can view the changes in tissue biochemistry and the changes in the IR frequencies in the tissue. This allows for a better visualization of the changes in tissue morphology because different tissues have different frequencies.
Using IR-OH hybrid microscopes we can view the changes in tissue morphology and the changes in the tissue biochemistry at the same time, resulting in a more accurate image of the tissue sample. The technique that we used was to image the specific vibration frequencies of the tissues. We would take 5000 frames at 600 Hz, then take 4 of those frames, demodulate them down to one image at that specific frequency. We did this at a range of frequencies and would slowly develop a complete image of the tissue covering a range of frequencies.
- Were there other optical cameras you considered, and why did you choose the Adimec Q-2HFW?
I learned about the Q-2HFW from the Adimec blog post of an interview with an MIT professor.
We knew we needed a camera with full well pixels but the signal to noise ratio from other cameras that we looked at ended up being pretty bad. There were some cameras that met the requirements we needed, but they only had the ability to stream to internal memory and we needed to be able to stream the images from the camera to a computer and process it in real time, which is why we went with the Q-2HFW. We ran the camera for 7 days uninterrupted and ended up with 1000 tb of data that needed to be processed which is why we need the ability to stream to a computer in real time.
- Can we explain further why absorption of IR light by common optical imaging components makes mid-IR light incompatible with modern optical microscopy and almost all biomedical research and clinical workflows?
The idea for IR-OH imaging has been around since 2010 but it was thought that the signature noise was not going to be strong enough. A very bright light source is needed for the imaging process and with other cameras, the pixels would become too saturated. With the Q-2HFW we were able to achieve this imaging because even with a direct light shining onto the camera, it wasn’t possible to over saturate the sensor due to the deep pixels. Also, we didn’t lose image quality. We bought the brightest LED bulb I could find, and we couldn’t saturate the camera, which was key to our process.
- What internal firmware / software features specific to the Adimec Quartz camera helped with the microscopy imaging?
In terms of internal hardware, we just used the raw frames with nothing like flattening or anything like that. We did, however, use external triggering on the camera.
- How do you see this technology growing and being adopted more by the pathology community?
IR-OH is compatible with both research and clinical pathology practices and could provide a cheaper alternative to the common stain-based methods that are in use today. In the clinical setting, they primarily use optical microscopes. These optical microscopes can only see the morphology of tissues but with the introduction of IR-OH microscopes, clinics will be able to study both the morphology and the biochemical composition of the tissue. In addition to providing this new way of imaging tissue in a clinical setting, this method is easier to apply, which will cut down on time and labor. The IR-OH method can change how research and clinical pathologist handle, image and understand tissue composition and structure.
This paper was published in The Proceedings of the National Academy of Sciences of the United States of America. Martin Schnell used the Adimec Q-2WFW-CXP camera to achieve the research done in this paper. The 2 Megapixel CoaXPress camera (Q-2HFW-CXP) brings a 1440×1440 resolution at up to 550 fps based on 12 micron pixels. Its global shutter CMOS image sensor is optimized for maximum full well performance allowing to capture very small signal changes in bright scenes. When compared to other high-framespeed CMOS sensor technologies available today (reference: 20 kel full well), the Q2HFW’s full well capacity (FWC) of over 2 million electrons per pixel is quite unique at around 100 times higher, delivering a unique SNR performance up to 63dB. The camera was developed as part of the FP7 funded CAReIOCA consortium.
The article that Martin Schnell wrote can be viewed here: https://www.pnas.org/content/117/7/3388
 English
English 日本語
日本語 简体中文
简体中文




